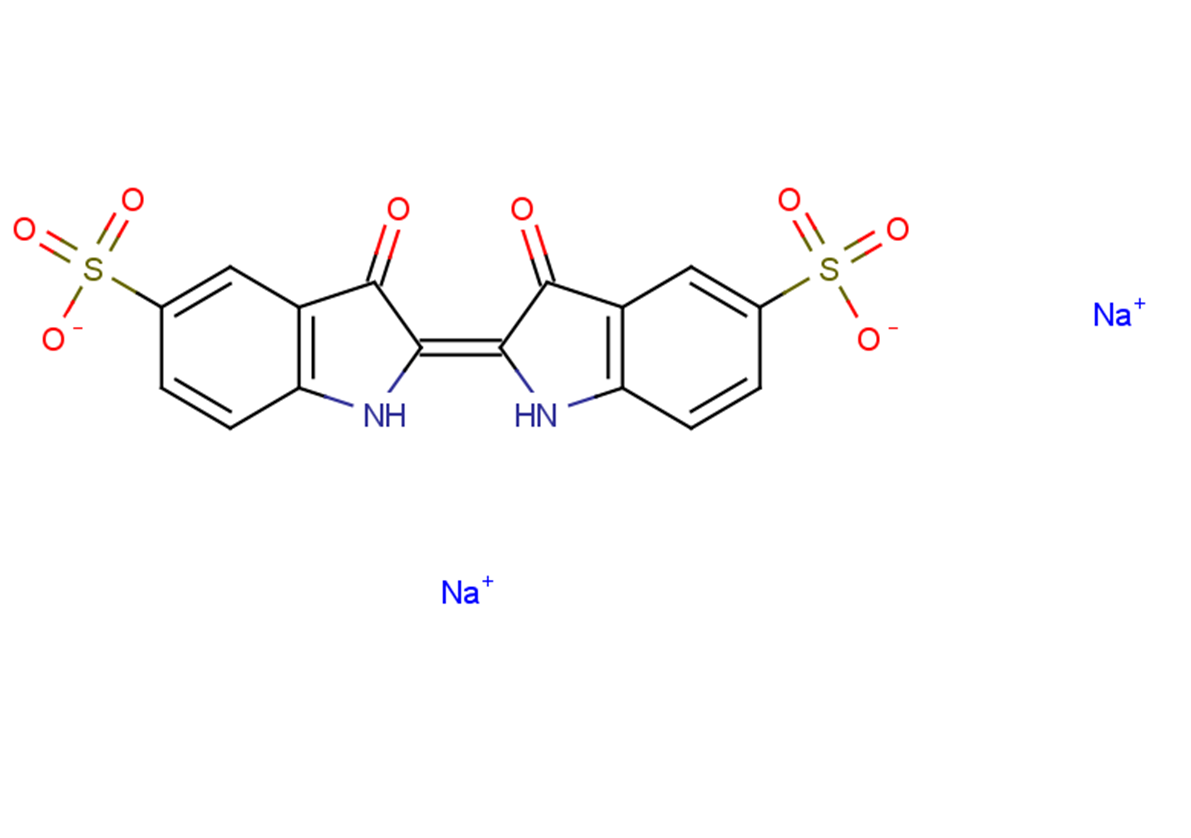
Indigo carmine
CAS No. 860-22-0
Indigo carmine( Indigotindisulfonate sodium | NSC 8646 | NSC-8646 | NSC8646 | Brilliant Indigo )
Catalog No. M24855 CAS No. 860-22-0
Indigo carmine is an indolesulfonic acid. It is used as a dye in renal function testing for the detection of nitrates and chlorates.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 500MG | 37 | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameIndigo carmine
-
NoteResearch use only, not for human use.
-
Brief DescriptionIndigo carmine is an indolesulfonic acid. It is used as a dye in renal function testing for the detection of nitrates and chlorates.
-
DescriptionIndigo carmine is an indolesulfonic acid. It is used as a dye in renal function testing for the detection of nitrates and chlorates.
-
In VitroIndigo carmine in a 0.2% aqueous solution is blue at pH 11.4 and yellow at 13.0. Indigo carmine is also a redox indicator, turning yellow upon reduction.
-
In Vivo——
-
SynonymsIndigotindisulfonate sodium | NSC 8646 | NSC-8646 | NSC8646 | Brilliant Indigo
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number860-22-0
-
Formula Weight466.35
-
Molecular FormulaC16H8N2Na2O8S2
-
Purity>98% (HPLC)
-
SolubilityH2O:18 mg/mL (38.60 mM; Need ultrasonic)
-
SMILESO=S(C1=CC2=C(N/C(C2=O)=C3NC4=C(C=C(S(=O)([O-])=O)C=C4)C\3=O)C=C1)([O-])=O.[Na+].[Na+]
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Herway ST, Pollock K, Fairbanks KE, Benumof JL. Diffuse Limb Discoloration with Indigotindisulfonate (Indigo Carmine?) and the Associated Implications. A A Case Rep. 2016 Apr 1;6(7):196-8.
molnova catalog



related products
-
Blumenol B
Blumenol B is a natural product of Rosa, Rosaceae. Blumenol B can be used as a reference standard.
-
Harmaline hydrochlor...
Harmaline hydrochloride?is a?fluorescent?indole?alkaloid?from the group of?harmala alkaloids?and?beta-carbolines.
-
2-O-Galloylmyricitri...
2''-O-Galloylmyricitrin is a natural product for research related to life sciences.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


